Ammonia work by the CEE group is led by Prof. A. Tsolakis, a.tsolakis@bham.ac.uk
Ammonia is a promising carbon-neutral fuel for internal combustion engines. Aside from boasting zero carbon dioxide emissions, it offers several advantages: high hydrogen content, high energy density, low flammability, excellent octane rating, and safe storage. However, before widespread adoption in applications like the transportation sector, some technical and logistical hurdles need to be tackled.
One key challenge lies in ammonia’s combustion characteristics. Its low flame speed, wide flammability range, and low ignition temperature pose engineering difficulties. Researchers have been exploring solutions like catalyst assisted combustion, staged combustion, oxy-fuel combustion, mixed ammonia-hydrogen blends, advanced combustion chamber designs, and even plasma-assisted combustion to overcome these limitations.
It is worth noting that while ammonia eliminates carbon emissions during combustion, it introduces other challenges including nitrogen oxide (NOx) emissions and unburned ammonia escaping from the combustion chamber (ammonia slip). Controlling those emissions is under investigation by both in-cylinder techniques through improvements in combustion efficiency and effective aftertreatment solutions.
The University of Birmingham’s Clean Energy Engineering Research Group (CEE-UoB), which is active in the MariNH3 project, is working on both fronts: exploring ways to improve the combustion of NH3 and to reduce emissions by investigating new catalytic exhaust aftertreatment systems.
We are evaluating various scenarios for using ammonia in transportation engines, both as a sole fuel and in dual-mode configurations with traditional fuels. Our research focuses on finding optimal configurations and conditions that enable stable operation, minimal engine modifications, and zero equipment damage. We are also exploring various catalyst configurations, both commercially available and novel prototypes, to ensure this fuel adheres to existing stringent emission standards, while anticipating potential future adjustments to these regulations.
Hydrogen, ammonia, and ammonia/hydrogen combustion engines
Of the techniques mentioned above for improving the combustion characteristics of ammonia, one of the most promising is the addition of hydrogen.
Introducing small quantities of hydrogen into the fuel mixture can yield noteworthy improvements due to its unique combustion characteristics. This “simple” addition dramatically improves ammonia’s combustion characteristics by increasing combustion rate, extending flammability limits, enhancing flame stability leading to reduced carbon-based emissions and higher energy efficiency (Chai, et al. 2021).
In preparation for our ongoing ammonia/hydrogen with another liquid fuel experiments, we conducted an initial investigation to assess the tangible effects resulting from the combination of hydrogen and gasoline. Our primary focus was on evaluating its stability under poor lean conditions and understanding its influence on emissions. The findings have been documented and presented by Yavuz et al. (2024). Figure 1 provides a glimpse of the results.
Figure 1. In-cylinder pressure with respect to the crank angle for 0% and 12% H2 energy share.
When some of the gasoline is replaced with hydrogen in the engine, the in-cylinder pressure it generates when burning the fuel increases.
Hydrogen’s lower energy density requires more intake volume, leading to higher initial pressure. Additionally, its faster burning rate shortens ignition delay and increases pressure rise, amplifying peak pressure in the cylinder.
In short, hydrogen fuel gives the engine’s pressure a boost and burns faster, all thanks to the hydrogen properties.
Hydrogen is known to produce these effects as it has a combustion rate 10 times faster than gasoline, resulting in a more complete and efficient combustion, which translates into higher fuel efficiency. Hydrogen also mixes more easily with air than gasoline or diesel (due to its better diffusivity), creating a more homogeneous mixture, resulting in more uniform combustion, and reduced carbon-based emissions (CO, CO2).
Hydrogen can be used to improve the combustion of ammonia precisely because of the properties described above. It can be injected as a third party (with ammonia and gasoline), premixed with ammonia or, even better, it can be created from ammonia with some ingenuity. The latter allows for the utilisation of the energy that is wasted with the exhaust gases, increasing the efficiency of the overall process. Interestingly, this connects to another project developed by CEE-UoB (Sittichompoo, et al. 2021).
At CEE-UoB, we are conducting experiments in this direction, under a wide range of conditions and in different types of engines. Specifically, we have been investigating the effect of partially substituting gasoline with H2/NH3 mixtures. Our goals include exploring how far we can go with lean burn conditions without compromising the stability of the combustion process and, above all, controlling emissions. Figure 2 shows a glimpse of a work in progress.
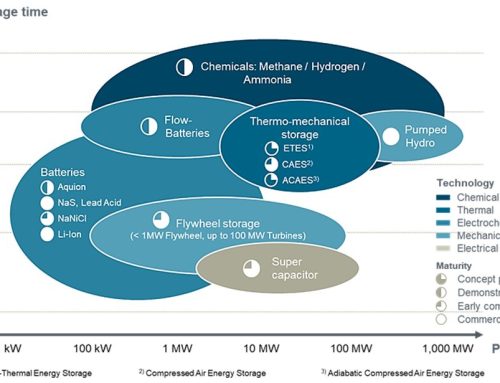
Figure 2. In-cylinder pressure with respect to the crank angle for gasoline and gasoline + 30% H2/NH3 energy share
The figure demonstrates the effect of substituting 30% of gasoline, in terms of energy, with an H2/NH3 mixture in a spark ignition (SI) engine operating under identical performance conditions. The observed increase in maximum pressure within the piston implies improved engine efficiency. Most importantly, this substitution of hydrocarbon-based fuels with a zero-carbon alternative like the H2/NH3 mixture directly contributes to reducing CO2 emissions.
However, using H2 as combustion improver also presents challenges, such as NOx production due to the high temperatures generated during combustion. Various strategies are being investigated to address these challenges, almost all of which fall under one of the following approaches: combustion optimisation and development of new after-treatment strategies (Zhu, et al. 2024).
Remember I told you that one of the biggest challenges was NOx production and ammonia slip? Advances in these areas could enable the efficient and clean use of ammonia as a fuel, with a significantly lower environmental impact than traditional fossil fuels. We are also working in this direction.
Combustion modelling
Virtual testing through combustion modelling has advantages compared to complex real-world combustion testing in terms of speed, safety, and economy (although, we must not forget what George Box said about the models!).
By simulating chemical reactions, heat transfer, and fuel consumption, we can gain profound insights, optimise processes, and develop cleaner and more efficient technologies on fuel combustion, and even in aftertreatment processes. Kinetic-chemical simulation is a powerful tool. This methodology harnesses the concept of fuel virtualisation, utilising published kinetic-chemical mechanisms and robust mathematical models.
Under controlled conditions, this approach enables the replication of experimental results with quantifiable uncertainties. By revealing the complexities within the combustion chamber, it not only provides explanations for observed phenomena but also enables the prediction of behaviour under diverse operational scenarios. More specifically, we may know how to improve the efficiency of the combustion process, even how to produce fewer harmful emissions.
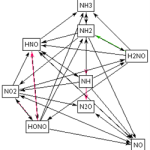
Figure 3. Reaction pathway diagram of ammonia oxidation
At the CEE-UoB, we strive to optimise internal combustion engines through advanced modelling techniques. We are focussing on achieving efficient combustion with ammonia as the sole fuel and developing strategies to partially replace conventional fuels (gasoline or diesel fuel) with ammonia.
To achieve these goals, we leverage both cutting-edge commercial software and our own custom-developed tools which allows us to tailor our simulations to specific needs and gain deeper insights into the engine’s combustion process. Furthermore, we boast state-of-the-art engine facilities equipped with advanced sensors and instrumentation which enables us to precisely control key operating variables and compare experimental data with our simulation results, ensuring the accuracy and validity of our models.
We are interested in understanding the chemical kinetics of ammonia decomposition during combustion, focusing on oxidation under lean conditions and the formation of nitrogen oxides (NOx: NO and NO2). The reactions involve complex interactions between NH3, O/OH/H radicals, and intermediate species such as HNO, HCN, and N2O. Figure 3 shows a simplified representation of the process.
The figure shows a “snapshot” of the sequence of reactions that occur during the formation of NO from NH3 (fuel-NOx). The complexity of the process is evident, even though it is very simplified. However, it allows to identify preferential pathways, branching rate, etc.
To conclude, we are still working on the preparation, generation and interpretation of our results. Our goal is to offer refined results, correctly presented, and commented in high-level publications. In this way, the CEE-UoB contributes to building a cleaner world.
Bibliography
- Chai, Wai Siong, Yulei Bao, Pengfei Jin, Guang Tang, and Lei Zhou. 2021. “A review on ammonia, ammonia-hydrogen and ammonia-methane fuels.” Renewable and Sustainable Energy Reviews 111254. doi:10.1016/j.rser.2021.111254.
- Sittichompoo, S., H. Nozari, J.M. Herreros, N. Serhan, J.A.M. da Silva, A.P.E. York, P. Millington, and A. Tsolakis. 2021. “Exhaust energy recovery via catalytic ammonia decomposition to hydrogen for low carbon clean vehicles.” Fuel 119111. doi: https://doi.org/10.1016/j.fuel.2020.119111.
- Yavuz, M., G. Brinklow, A. Cova Bonillo, J. M. Herreros, D. Wu, A. Tsolakis, P. Millington, S. Alcove Clave, O. Doustdar, and S. Zeraati-Rezaei. 2024. “The Suitability of the Three-way Catalyst for Hydrogen Fuelled Engines.” Johnson Matthey Technology Review. doi:10.1595/205651324X17054113843942.
- Zhu, Xuren, Jianguo Du, Zhou Yu, Yi-Bing Cheng, and Yu Wang. 2024. “NOx Emission and Control in Ammonia Combustion: State-of-the-Art Review and Future Perspectives.” Energy and Fuels 43–60. doi:10.1021/acs.energyfuels.3c03104.